CoE Programme and Procedure
Here you can find detailed information about the certification programme from center training to re-certification.
(Please find the download center by scrolling down the page.)
NEWS: Updated ENETS CoE Policy
ENETS has updated its ENETS CoE policy for extra-EU centers applying for accreditation in 2023/24! Please read the policy update, especially if you are an extra-EU center looking to apply for the ENETS CoE accreditation programme from 2023/24 onwards.
1. Center Training
- Center training is free of charge and should be passed in the year (not longer than 2 years) prior to the application.
- Training is held either physically during the ENETS Annual Conference, or virtually in Q1 of the year.
- In physical meetings, participation is limited to two representatives per center. (More representatives may be able to attend last minute depending on registration numbers and capacity. Please enquire at the ENETS Office - info@enets.org.) Presentations will be made available as a pdf file.
- Upcoming dates: center training workshop dates will be displayed here in Q4 of each year. Click the button below and don’t miss any important dates.
2. CoE Application
Update to the ENETS CoE policy re. non European ENETS CoE applications in 2023/4!
ENETS has updated its policy with respect to non European ENETS CoE applications for non European centers looking to apply for ENETS CoE accreditation in 2023/4 and beyond - applications for new ENETS CoEs will only be open to European centers (including United Kingdom) over the next 3-5 years. (This change in policy will not affect currently accredited extra-EU CoEs in Australia, Israel or the United States.)
The applicant or center representative must be an ENETS member. Please click here if you are not yet an ENETS member.
All centers wanting to apply for certification must fulfil the requirements as outlined in the ENETS Center of Excellence specifications which comprise:
- Governing structure
- Expertise on NEN multidisciplinary specialists qualifications (individual qualifications and NET-related expertise) with access to resources, have quality-related processes, and performance data
- Interdisciplinary cooperation and communications structure
- Specialist NEN consultation with coordination of patient flow
- Multidisciplinary tumour board meeting
- Patient involvement
- Scientific activities
- Follow-up and tumour documentation (NEN-related data base)
- Quality indicators and key figures (became a mandatory annual return from 2014).
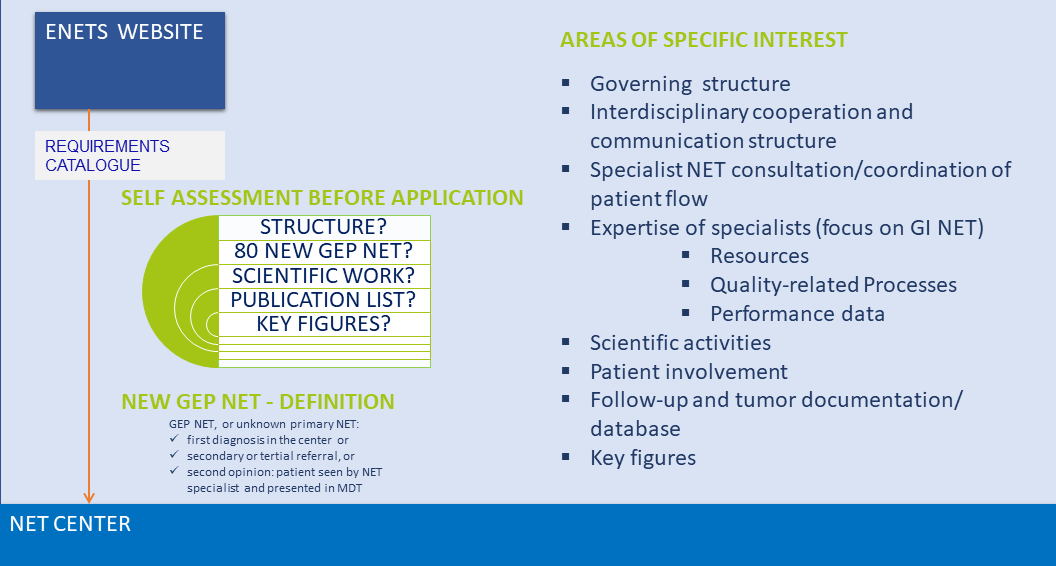
Please note the general threshold for application is 80 NEW GEP NEN patients per year. The threshold for pulmonary NEN is 20 NEW PULM NEN per year.
The application contains information on quantitative and qualitative issues pertaining to the individual centers and the assessment is done independently of ENETS.
In general, a GEP NET center/ network should consist of one or not more than three clinically collaborating hospitals/ locations. The physical distance between the sites should should still allow for meaningful collaboration and clinical management.
Centers not falling under the criteria outlined above could be considered as “affiliated” partners and not as part of a certified multi-site center. Some limited exceptions can perhaps be made for a very specific indication such as a specific therapy/test and at the discretion of the ENETS Certification Commission / ENETS Executive Committee.
In general, a CoE could evolve over time, become a local HUB and develop a network of affiliated centers, they relate to.
If a network or group of centres are applying, the overall structure requires detailed explanation at the outset and should be clarified during the application process. A clear and proven daily- work practice/collaboration and structure within groups has to be in place. Collaboration simply to increase patient numbers is not acceptable; the structure has to be a functioning unit.
Clarification on protection of status quo: Existing multisite CoE are subject to the rules as they were at the time of their initial certification.
3. CoE Enrolment
All applications undergo a thorough two-step assessment and are individually discussed by the ENETS Certification Commission.
Desktop audit: A desktop audit will be conducted by the ENETS Office to ensure completeness of the application. Contact will be made with the applicant if information is pending or remains unclear.
Decision on enrolment: After thorough discussion, a decision will be taken by the ENETS CC on enrolment. Contact will be made with the applicant if information is pending or remains unclear.
The decisions are taken unanimously.
- Involving the certification company – tailoring the individual audit programme of the year.
- Certification contract between CoE and certification company.
- Communication between lead auditor and two representatives of the CoE applicant (clinical lead or center coordinator plus administrative coordinator.
- Planning the on-site audit (including travel).
- Agreement on date of submission of the “file of evidence”.
4. Audit Procedure
Deadlines
The centers selected for the accreditation process (initial as well as re-certification) are obliged to provide the completed CoE documentation 9 weeks prior to the scheduled audit date.
It is the responsibility of the center to meet all deadlines. Missing the deadlines means in effect quitting the procedure. Fees will not be refunded, and any travel expenses accrued must be covered/paid by the individual center.
The file of evidence
- The requirements catalogue, filled in with individual center information in the right column or equivalent documentation.
- Contracts/agreements as PDFs
- Certificates of expertise, i.e. PDFs of CV, with a focus on GEP NET
(E.g. for pathologists: Senior pathologist (5 years of experience); participation in a course in NET pathology (or similar) as proof of expertise, proof of participation in ENETS meetings.
- Task descriptions (data manager, quality manager, head of the center, and all others itemised in the catalogue)
- Detailed information on patient flow (who sees the patient first, who coordinates diagnostics and treatment, who collects follow-up data?)
- Detailed information about the tumor board, including proof of attendance of the required NET experts from the main partner disciplines and information on treatment decision
SOP as defined as mandatory in the requirements (pathology reporting, PRRT, interventional radiology)
- Updated data (chapter 15 requirements catalogue)
- Updated anonymised patient list
- Updated list of patients in clinical trials
- Updated publication list
- Analysis of waiting times (mandatory for re-cert audits)
- Analysis of patient feedback (mandatory for re-cert audits)
Audit plan
- The audit plan will be drafted according to the provided documentation and anticipated need for discussion time - this means that the timeslots for department visits can vary. The auditors expect to witness a real-life NEN tumor board (Multidisciplinary Team Meeting – MDT).
- Final audit plans are sent to the individual center four weeks prior to the scheduled audit date.
On-site audit
- Each audit is conducted by at least two auditors, one ENETS expert auditor and one GSG auditor.
- The audit time required is 7-8 hours - this allows some flexibility and the audit plan, if necessary, will be arranged either in accordance with the center's (time) preference or with the auditors once they are on-site.
- At the end of the audit, the auditors will provide feedback and a recommendation on whether the certificate should be awarded. However, the final decision will be made by the Certification Commission.
Audit report
The two on-site auditors will write up the audit report. The audit report will be revised by another independent expert auditor (desktop auditor).
Decision
- Provided the desktop auditor agrees to the on-site auditor recommendations, the audit report will then be sent to the center representative, and a certificate can be issued by the certification company.
- In case there are still open questions between the on-site auditors and the desktop auditor, the ENETS Certification Commission will convene and discuss the audit findings. The ENETS Certification Commission will take the final decision on awarding the certificate. DQS will inform the respective center about the final decision via e-mail.
Appeal
Each center has the right to appeal a decision made by the ENETS Certification Commission. The center itself is responsible for formulating and lodging an appeal. All appeals will be discussed by the ENETS Certification Commission and the ENETS Executive Committee.
Preparation of certificates
In order to issue the official certificate, the individual center will be requested to provide its logo, official name and address. Certificates will be sent by registered post once all administrative and financial processes have been completed.
Acknowledgements
New ENETS CoEs will be acknowledged during the Annual ENETS Conference and on the ENETS website.
Auditors
We would like to introduce our Auditors:
5. Structure
Issuing body of the certificate
ENETS as issuing body takes responsibility for developing the ENETS CoE requirements catalogue and certification programme.
Independent certification company
The certification process is undertaken and completed by an independent certification company. DQS is ENETS' current partner.
DQS GmbH
August-Schanz-Straße 21
60433 Frankfurt am Main
Independent auditors
Each onsite audit is conducted by at least two auditors, one ENETS expert auditor and one DQS auditor. The ENETS CoE Certification Commission may decide to delegate more expert auditors, e.g. for multi-site centers.
ENETS CoE Certification Commission
The Certification Commission consists of:
- All ENETS expert auditors
- Chair of ENETS CoE Programme
- Representative of the Certification Company
Tasks
- Deciding which CoE applicants may enrol in the certification programme.
- Recommending whether individual certificates should be awarded.
- Supporting the further development of the ENETS CoE requirements catalogue and certification programme.











